November, 06 2018 - Seeing the invisible
November, 05 2018 - The science of reading in science: A never-ending story
October, 16 2018 - To kill or not to kill
May, 15 2018 - Armoring the body against cancer
November, 06 2018
Seeing the invisible
Of course, this is how we see it from our perspective. If we appeared in the body of an ant, we would see humans as giants and indeed the world around us would feel much different. And we can go smaller than an ant! Thanks to our eyesight we are able to see details as small as 0,1 mm. And mother nature is by far not limited by this.
 The “cosmos of small things” was observed for the first time by a Dutch textile seller, Antoni van Leeuwenhoek. He was using magnifying lenses to check the quality of linen. Luckily, linen was not the only thing he looked at. With further improvement of lens quality and magnification he discovered microorganisms, blood cells, muscle fibers, sperm and many other microscopic structures. He invented the microscope, and with it, he made a completely new universe – a very near one – reachable for us.
The “cosmos of small things” was observed for the first time by a Dutch textile seller, Antoni van Leeuwenhoek. He was using magnifying lenses to check the quality of linen. Luckily, linen was not the only thing he looked at. With further improvement of lens quality and magnification he discovered microorganisms, blood cells, muscle fibers, sperm and many other microscopic structures. He invented the microscope, and with it, he made a completely new universe – a very near one – reachable for us.
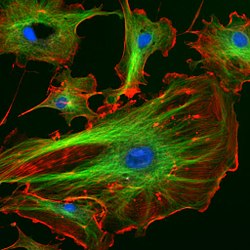 So how can we solve this problem? A possible solution lies in what we know as fluorescence microscopy: a special type of light microscopy where we can tag the structures or even molecules that we want to observe with a “light” label. Then we activate the labels with a laser and record their positions. In this way we can study the movement, organization or even interaction of such labeled structures.
So how can we solve this problem? A possible solution lies in what we know as fluorescence microscopy: a special type of light microscopy where we can tag the structures or even molecules that we want to observe with a “light” label. Then we activate the labels with a laser and record their positions. In this way we can study the movement, organization or even interaction of such labeled structures.
MSc. Lukáš Veľas
Institute of Applied Physics
Vienna University of Technology
Member of the ENACTI2NG consortium
2nd image from Wikipedia, the free encyclopedia
November, 05 2018
The science of reading in science: A never-ending story
From Martel’s bioluminescent pools to Dante’s infernal hellscapes - books introduce me to the wonders of the world around me, and to those within my mind(s). From fire extinguisher labels (PASS!) to the Mahabharata’s godly discourse alike, reading is not merely a bedtime ritual for me, but a compulsive, relentless habit; a therapeutic necessity that helps me bridge the elusive gap between what I know, and what I am yet to learn. It’s an itch that I always scratch.
Starting a PhD gave me the perfect opportunity to cement my status as a bona fide bookworm. An afternoon well spent involves chasing down protocols, jumping from paper to paper, reading page after page, till my eyes tire, till my tummy rumbles - not stopping until my curiosity is satiated. Schreiber, Stagg and Allison adorn my lab bench and nightstand; my imagination and dreams.
And yet, I feel like I have not “read a book” in the last couple of months.
I’ve read a lot – but not novels or anthologies of poems. Instead, I’ve exclusively read myriads of papers and scientific articles in immunology, cancer biology and biochemistry. Don’t get me wrong, I’ve enjoyed every minute of it, and the scholastic accomplishment I’ve gained is unparalleled. Yet somehow, in my mind, I feel like I haven’t “read”.
So, why do I feel this way?
Papers and novels both equally enthrall me; however, science reports discoveries, but fails to narrate them to me. The unique identity and beauty of the stories scientists yearn to recite is masked and standardized by white sheets of A4 paper and rigid writing formats of science. A ten-page paper never allows me enough time or agency to invest in its story, to immerse myself into the narrative and let my thoughts marinate and linger. I feel like I have not “read” because scientific writing formats do not allow me to internalize what the paper posits the same way I would relinquish myself over to the story of The Boy Who Lived. The moment I meticulously dissect a graph, or get inspired by a novel protocol, constraints of the format divert my attention to another topic, or worse, summon a brusque conclusion of the paper. I miss the quiet pause of turning over a page of a novel. It gives me time to comprehend, condense and calibrate the last dozen pages’ information in preparation for the next dozen. Reading a paper feels like sprinting: I hold onto all the facts the best I can, juggling multiple trains of thought, processing them as fast as I can, while simultaneously being introduced to new facts and information at the same time. By the time I finish, I feel exhilarated and accomplished, but I am always out of breath.
What I miss even more is the physicality of reading a book – scrolling through an article on my computer screen doesn’t pose the satisfying struggle of contorting my fingers into obtuse angles to fit a fat book into. Every paper I download feels no different from the last one. Tepid, freshly printed pages sit limp in my hands. No dog-ears or spines broken into to fit the shape of my palms. No linty residue left on my fingertips from powder-yellow pages. No musty smells nor coffee stains that recount its previous lives and muses. Even though what I currently read is greater in volume and diversity compared to my routine reading list, my thirst for wallowing in the satisfaction of reading a book till its last page and closing it shut to add it to my bookshelf remains unequivocally unquenched. For me, the act of reading extends beyond the definition of the word, and the physical, mechanical ritual of interpreting and unraveling the words off of a book held in my hands seems to be an integral part of this experience.
I have come to speculate that I feel like I haven’t “read” because of the intrinsic non-resolvable nature of scientific narratives, a property that science and experimentation as fields at large also share. No scientific narrative can ever have an uncontestable, resolved ending. No happily-ever-afters, but rather to-be-continueds, and to-be-challengeds. More queries are generated than questions answered, giving papers a sense of being complete and incomplete at the same time. A sense of insufficiency, that possibly doesn’t quite satisfy the classical conciliatory needs of a reader.
Yet, reading in science feels like watching a good movie that never ends; it’s addicting nature fuelled by authors that never seem to stop writing. And as long as they keep writing, I will keep reading with a voracious appetite that seems to grow bigger and bigger, day after day, because the more I read, the less I seem to know.
MSc. Ashwathi P Menon
Aptamers
Center for Applied Medical Research
Member of the ENACTI2NG consortium
This email address is being protected from spambots. You need JavaScript enabled to view it.
October, 16 2018
To kill or not to kill
The human immune system has developed mechanisms to protect the body from various invaders, such as bacteria, viruses or parasites, which means we can fight off conditions like colds, the flu or food poisoning. Sometimes, however, the danger originates from within the body, as in the case of cancer. Cancer is a result of healthy cells undergoing changes, called mutations, which make them lose their natural function and divide uncontrollably.
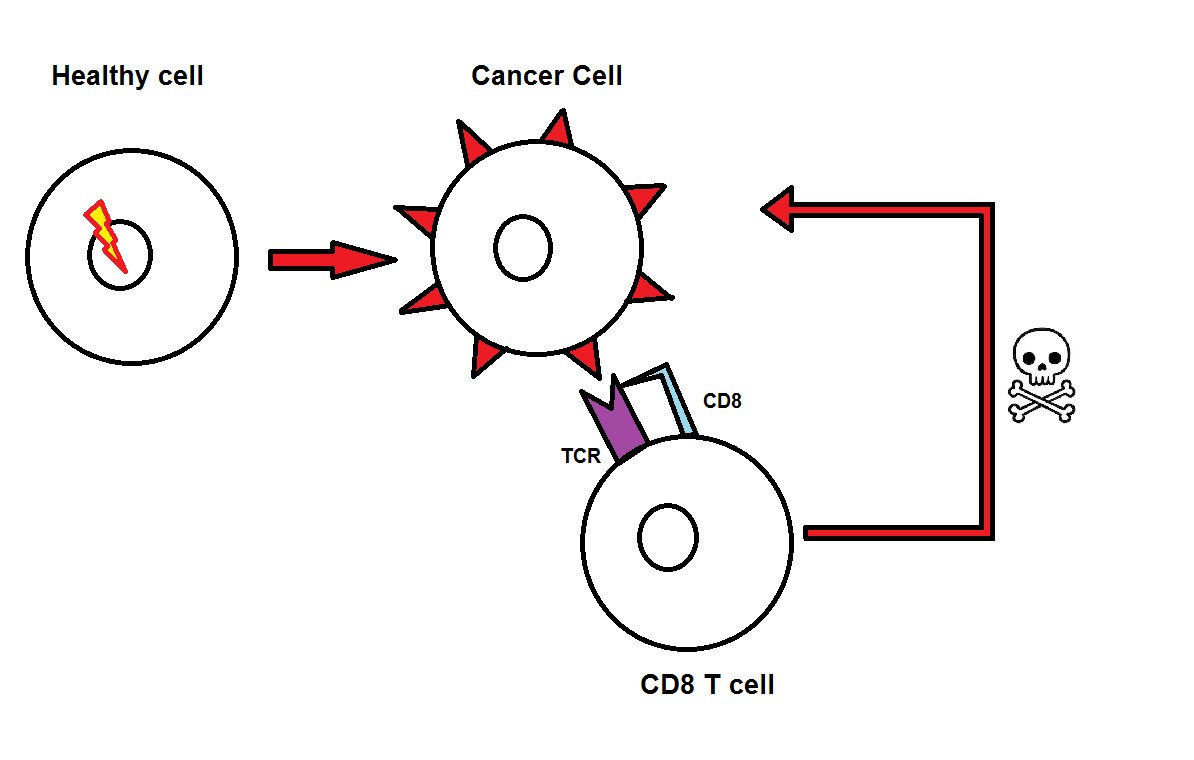
Since cell mutations are relatively common, the immune system also learnt to recognise healthy self from mutated self. The immune system consists of many cell types with various functions. One of these cell types is the CD8 T cell. It can destroy cancer by recognising patterns present on surface of cancer cells, which are often a result of the cell’s mutations. T cells recognise cancer through a molecule called TCR, which is present on their surface. Through a molecule present on their surface, called the TCR, T cells recognise cancer cells. Each TCR recognises one particular pattern. As a result of their mutations, cancer cells can carry either mutated patterns (natural patterns that would not normally be present at that stage of the cell’s life) or normal patterns (regular patterns that exist in higher amounts than on regular cells). All these cases can potentially activate T cells containing this pattern-specific TCR. However, cancer cells can carry mutated patterns common for other cell types, and the immune system classifies these patterns as “self”, and therefore harmless. This means that when a T cell with a TCR highly specific for this pattern was created, it was silenced or even destroyed by the immune system, and only T cells with less reactive TCRs were kept alive. In the battle against cancer, this puts cancer at an advantage, since the cancer-specific T cells are not very good at recognising and acting against cancer cells.
Nonetheless, this mechanism, with its strengths and defects, still exists in our body, and scientists have thought it possible to improve and use it as anti-cancer therapy. As Author described in a previous post, one such treatment is CAR therapy. Thanks to this, we can use T cells as the effector cell, but the TCR is partly replaced with a more powerful receptor. And while CARs have shown great promise so far, part of the research has focused on simply improving the TCR molecule itself. The idea behind TCR therapies is to find T cells containing cancer reactive TCR in patients with cancer and engineer these TCRs in the lab. Then, these reactive TCRs could be placed in the same patient’s T cells, or into T cells of patients that have no cancer specific TCRs.  Once expanded, the T cells would be returned to the patient and left to fight cancer. So far, this strategy has shown to reduce cancer load in several different studies, but compared to CARs, these T cells are less effective and don’t tend to survive long in the patient’s body. On the other hand, unlike CAR T cells, TCR engineered T cells can also recognise cancer patterns that are not necessarily present only on the surface of the cells, but also internally, making the range of possible cancer targets much broader.
Once expanded, the T cells would be returned to the patient and left to fight cancer. So far, this strategy has shown to reduce cancer load in several different studies, but compared to CARs, these T cells are less effective and don’t tend to survive long in the patient’s body. On the other hand, unlike CAR T cells, TCR engineered T cells can also recognise cancer patterns that are not necessarily present only on the surface of the cells, but also internally, making the range of possible cancer targets much broader.
In the Wooldridge T cell lab, part of our research focuses on improving current TCR therapies, but not through improving TCR molecules themselves. Alternatively, the aim is to improve a helper molecule present on T cells, called CD8 co-receptor, the function of which is to aid TCR recognition of patterns. With an improved CD8 molecule, the T cell could become more strongly reactive, even when the TCR is not highly sensitive to the cancer pattern.
Immunotherapies are putting human T cells into focus as main fighters of cancer. While CAR T cells show great promise in targeting several different types, TCR therapies are battling cancers that CARs cannot necessarily target. The human immune system has been fighting these battles all our lives, so no matter the strategy, current immunotherapies have great potential to help the body fight internal dangers by slightly improving the mechanisms that already exist.
MSc. Lea Knežević
Faculty of Medical and Veterinary Sciences
University of Bristol
Member of the ENACTI2NG consortium
This email address is being protected from spambots. You need JavaScript enabled to view it.
May, 15 2018
Armoring the body against cancer
For over a century, an extensive part of biomedical research has been focused on understanding and fighting cancer, involving thousands of oncology experts around the world. Common treatments such as surgery, chemotherapy and radiotherapy have increased and improved the life of millions of patients. However, they are highly invasive and expose the patients to external substances which are harmful not only to cancer cells, but also to healthy ones. What if we could fight cancer from the inside instead of from the outside? This is exactly what the foundation of immunotherapy relies on. It strengthens the immune system and makes it capable to fight and suppress cancer.
Within immunotherapy, there is a novel type of treatment that’s having increasing success in clinical trials with patients suffering from a blood cancer type called leukemia. This treatment involves a modified immune cell called Chimeric Antigen Receptor (CAR) T cell.
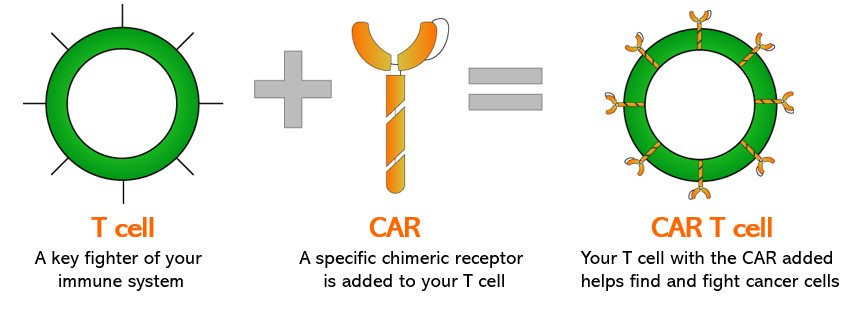

One of the remarkable examples of the successful experiences with this therapy is the case of Emily Whitehead. She suffered from leukemia in an advanced stage with low survival expectancy. Emily was the first person to receive the CAR T cell therapy. She had the quickest response the doctors had seen in the shortest period of time. Emily has been free of cancer for more than 5 years.
Notwithstanding, if this therapy is so promising, what are the challenges now? Several, because unlike Emily, other patients have presented dangerous side effects as an overactivation of the immune system or alterations of the nervous system. The major ones are finding the balance between the activation and control of the immune system with the CAR T cells, discovering new targets to tackle in the cancer cells and making this therapy effective to other types of cancer besides leukemia. It is exactly in these points where a vast part of the research of immunology is focusing this year.
Multiple groups of experts around the world are addressing these challenges, like the European Network on Anti-Cancer Immuno-Therapy Improvement by modification of CAR and TCR Interactions and Nanoscale Geometry (ENACTI2NG). In this network, young scientists from around the world collaborate in universities located in Germany, Spain, the Netherlands, Austria and England, undertaking the challenges mentioned above.
One of the members of this consortium is the University of Freiburg in Germany, where the research is led by Professor Wolfgang Schamel and Dr. Susana Minguet. This project is attempting to modify the design of the engineered components in the CAR T cells, introducing new molecules that are related to the activation, survival and growth of the T cells. The idea is maintaining the activation of the immune system to fight cancer but at the same time reducing the side effects. During the next years, the first results will come to light.
Even though we still cannot predict how long it will take to design a therapy which can fight all types of cancer, the reality is that we are already living in a new era of cancer treatment: the CAR T cell therapy era.
MSc. Rubí Misol-Há Velasco Cárdenas
PhD Student on the improvement of CAR T cell therapy
Albert-Ludwigs-Universität Freiburg
Member of the ENACTI2NG consortium
This email address is being protected from spambots. You need JavaScript enabled to view it.
Images from Juno Therapeutics and Parker Institute for Cancer Immunotherapy.


